Exploring Synaptic Integration and Neuronal Communication in the Cellular Neurophysiology Laboratory
The fundamental role of nerve cells is to integrate their synaptic inputs to generate an output signal, known as the action potential. The lab aims to unravel the mechanisms behind how presynaptic nerve cells release neurotransmitters, how these neurotransmitters activate postsynaptic receptors, and how the resulting postsynaptic potentials are integrated to produce an action potential. The lab explores these questions through four key research areas using molecular, neuroanatomical, in vitro electrophysiological, imaging, and in silico modeling methods:
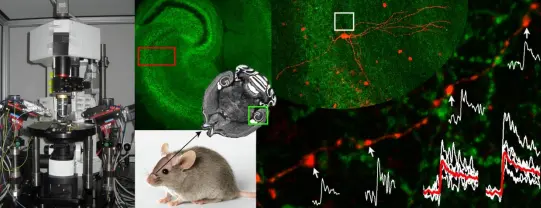
- Investigating the role of specific nerve cells in olfaction, using pharmaco- and opto-genetic techniques to modify olfactory nerve cell activity during odor discrimination tasks.
- Exploring the molecular, structural, and functional diversity of excitatory and inhibitory synapses in the cortex, focusing on transmitter release probability, short-term plasticity, and postsynaptic receptor activation. The lab combines in vitro electrophysiology, two-photon imaging, and light- and electron microscopy to analyze these synaptic properties.
- Mapping the neuronal surface by studying the location and density of voltage- and ligand-gated ion channels in distinct subcellular compartments of identified nerve cells, using quantitative light- and electron microscopy. Multi-compartmental modeling is applied to predict the functional effects of specialized ion channel distributions, which are then tested using electrophysiology and imaging.
- Providing a quantitative analysis of the microcircuit in the cerebellar cortex, focusing on electrically coupled GABAergic interneurons and their role in network functionality.
1. Understand the role of identified nerve cells in olfaction:
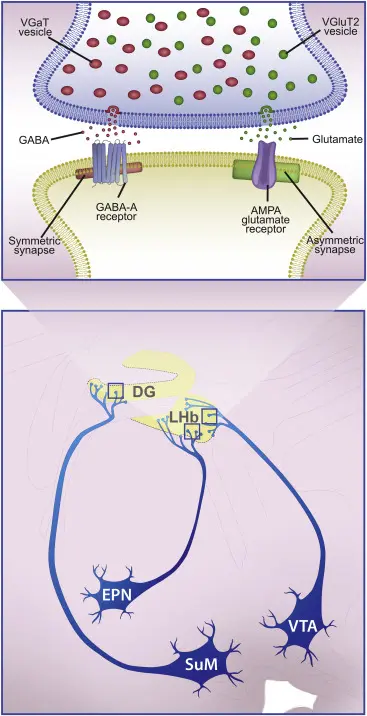
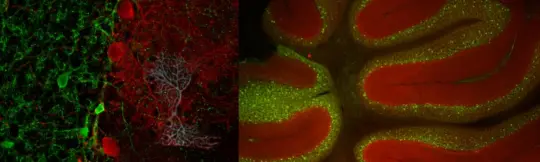
The extraordinary diversity of nerve cells was already recognized over a century ago. It is now widely accepted that within most brain regions,including the main olfactory bulb (MOB), glutamatergic principal cells are rather homogeneous, whereas GABAergic interneurons (INs) form a diverse cell population. The laboratory of Cellular Neurophysiology has identified
novel GABAergic IN subtypes in the MOB, which showed unique connectivity patterns. An interesting issue regarding the diversity of INs is identifying the role individual cell types might play in olfaction. Dr Nusser’s laboratory aims to reveal the role of distinct cell types of the MOB and other olfactory brain areas using pharmaco- and opto-genetic approaches. Currently, three different pharmaco-genetic methods are used in the laboratory: 1) a method to employ zolpidem to selectively potentiate GABAA receptor-mediated
synaptic currents in a specific subset of neurons (Wulff et al., 2007, Nat Neurosci), 2) designer receptors engineered from human M3 muscarinic receptors mutated to be exclusively activated by the drug clozapine-N-oxide (CNO; Alexander et al., 2009, Neuron), 3) a mutated nAChR and GlyR chimera, which is selectively activated by a designer drug (Magnus et al.,2011, Science). These designer receptors and the wild-type GABAAR2 subunit will be delivered to the brain with stereotaxic injection of adenoassociated viruses either in a cell type-specific or a non-specific manner. Two to four weeks after the injections, the animals will be trained in odor discrimination tasks either in a freely moving or in a head-restrained way. The effects of pharmacological silencing of certain neuronal populations will be tested on the behavioral performance of the animals. In the head-restrained behavioral task, the cells will be also silenced by the use of light-activated archaerhodopsin.
2.Investigating the Molecular and Ultrastructural Diversity of GABAergic and Glutamatergic Synapses :
Understanding chemical synaptic neurotransmission in the central nervous system (CNS) has been a central focus of neuroscience research for decades. Significant progress has been made in understanding the molecular processes that lead to neurotransmitter release from synaptic vesicles, the diffusion of neurotransmitters to their postsynaptic receptors, and the activation of these receptors. Simultaneously, ultrastructural analysis of synapses has revealed a remarkable diversity in the shape and size of pre- and postsynaptic structures across central synapses. Recent molecular studies have further identified numerous molecules involved in pre- and postsynaptic function, highlighting the molecular diversity of key components. However, the exact mechanisms that generate functional heterogeneity in synapses, as well as the impact of alterations in synaptic geometry and molecular content on synaptic function, remain unclear.
The Laboratory of Cellular Neurophysiology is combining in vitro electrophysiology and two-photon calcium imaging with electron microscopy to investigate the relationship between the diversity in release probability and short-term plasticity at different synapses, and the size and shape of presynaptic active zones. Additionally, quantitative electron microscopy with freeze-fracture replica immunogold labeling is used to reveal the molecular composition of structurally and functionally distinct active zones. These findings provide new insights into the structure-function relationship of central synapses, offering a molecular explanation for why larger active zones have a higher release probability at hippocampal CA3 pyramidal cell synapses. The results also support the hypothesis that ultrastructural differences among synapses within the same population (e.g., hippocampal CA3 pyramidal cell to CA1 pyramidal cell connections) reflect molecular specializations that contribute to distinct functional properties.
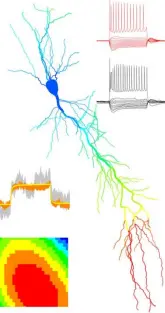
3.Mapping the Molecular Distribution of Ion Channels in Neuronal Surfaces
The primary function of nerve cells is to integrate synaptic inputs and generate action potentials (APs). Traditionally, it is believed that APs are generated in the axon initial segment (AIS), but synapses are often spread over the entire dendritic tree, meaning the distance between a synapse and the output site varies. This spatial arrangement leads to different filtering effects of postsynaptic responses by dendrites. If dendrites were passive, the effect of a synapse on action potential generation would depend solely on its location. However, research has shown that dendrites are not passive; they contain numerous voltage-dependent conductances that provide significant computational power.
The molecular identity, precise location, and density of voltage-gated ion channels in small subcellular compartments along the axo-somato-dendritic surface are crucial for synaptic integration and action potential generation. Past studies have provided broad conclusions on ion channel distributions, such as the Kv1.1 subunit being axonal or HCN1 being somato-dendritic. However, recent work using high-resolution immunolocalization has revealed that ion channel subunits show distinct distribution patterns across different neuron types, leading to more complexity in understanding their roles.
The axonal domain, for example, includes the AIS, nodes of Ranvier, myelinated axon segments, non-myelinated preterminal axon segments, axon terminals, and presynaptic active zones—each with specific functional roles that require unique ion channels. The lab's research has demonstrated that ion channel subunit distribution is highly specific to subcellular compartments.